Diazyme's Human Kappa Free Light Chain (FLC) assay has excellent across the Analytical Measuring Interval (AMI) in standard mode: 3.5 – 200 mg/L ; extended mode: 3.5 – 4000 mg/L. The method comparison gave a linear regression coefficient of 0.972, a slope of 1.054 and y-intercept of 0.636 mg/L.
| Product | Catalog Number | Format |
|---|---|---|
| Kit (Import) | DZ169A-K | R1/R2 (Dual Vial Liquid Stable, Immunoturbidimetric) Cal: 5 level (Included with kit) |
| Kit (Export) | DZ169A-KY1 | R1/R2 (Dual Vial Liquid Stable, Immunoturbidimetric) Cal: 5 level (Included with kit) |
| Control | DZ169A-CON | Con: 2 Level |
Product Features
Diazyme's Human Kappa Free Light Chain (FLC) assay has excellent linearity across the Analytical Measuring Interval (AMI) in standard mode: 2.9 – 150 mg/L; extended mode: 2.9 – 3000 mg/L. The method comparison gave a linear regression coefficient of 0.977, a slope of 0.958 and y-intercept of -2.536 mg//L. Precision of the assay was evaluated according to the CLSI EP5-A guidelines and has a Within-Run precision from 1.1% - 7.9% and a Total Precision of 1.7% - 11.7%. The Kappa assay displays outstanding sensitivity with an LOD of 1.8 (Export 1.4) mg/L; LOQ of 2.9 (Export 2.3) mg/L; and a LOB determined to be 0.9 mg/L.
Downloads
Informational Articles
- 2022 Wu Adequate Antibody Response to COVID-19 Vaccine
- 2022 Singh Serum Free Light Chain Quantification Testing
- 2022 vercammen serum free light chain analysis
- 2021 Russcher Method comparison of three serum free light chain assays on the Roche Cobas 6000 c
- Analytical and Clinical Concordance of Free Light Chain Assay
- Alan Wu Customer Testimonial
- New England Cancer Specialists Customer Testimonial
Assay Principle
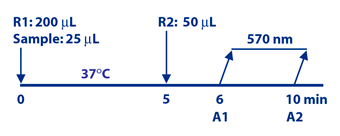
Diazyme Human Kappa Free Light Chain Assay is based on a latex enhanced immunoturbidimetric assay. Kappa FLC in the sample binds to specific anti-Kappa FLC antibody, which is coated on latex particles, and causes agglutination. The degree of the turbidity caused by agglutination can be measured optically and is proportional to the amount of Kappa FLC in the sample. The instrument calculates the Kappa FLC concentration by interpolation of obtained signal of a 6-point calibration curve prepared from calibrators of known concentrations.
Intended use
The Diazyme Human Kappa (κ) Free Light Chain Assay is intended as a latex particle enhanced immunoturbidimetric assay for the quantitative determination of Kappa Free Light Chain (FLC) concentration in serum on validated analyzers. The measurement of Kappa FLC in conjunction with Lambda FLC aids in the diagnosis and monitoring of multiple myeloma in conjunction with other laboratory and clinical findings. For in-vitro diagnostic use only.
Regulatory Status

 Health Canada Registered
Health Canada Registered

