Diazyme's Carcinoembryonic Antigen (CEA) Assay is a dual liquid stable reagent system which provides reliable results. The method requires 25 μL of sample, with test results completed in less than 10 minutes. The CEA assay has a Within-Run %CV of less than 4.63% and has a linear range up to 100 ng/mL. The liquid stable format requires no reagent preparation.
| Product | Catalog Number | Format | Method |
|---|---|---|---|
| Kit | DZ863A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ863A-CAL | Cal: 6 Level | |
| Control | DZ863A-CON | Con: 2 Level |
Product Features
Diazyme's Carcinoembryonic Antigen (CEA) Assay is a dual liquid stable reagent system which provides reliable results. The method requires 25 μL of sample, with test results completed in less than 10 minutes. The CEA assay has a Within-Run %CV of less than 4.63% and has a linear range up to 100 ng/mL. The liquid stable format requires no reagent preparation.
Downloads
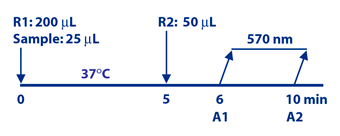
Assay Principle
Diazyme's CEA Assay is based on a latex enhanced immunoturbidimetric assay. CEA in the sample binds to the specific anti CEA antibodies, which are coated on latex particles, and causes agglutination. The degree of the turbidity caused by agglutination can be measured optically and is proportional to the amount of CEA in the sample. The instrument calculates the CEA concentration obtained signal of a 6-point calibration curve.
Intended use
The Diazyme CEA Assay is for the determination of carcinoembryonic antigen concentration in serum by latex enhanced immunoturbidimetric method.
Regulatory Status



