The Diazyme High Sensitive C-Reactive Protein (hsCRP) Assay has a linear range of 0.20 - 20 mg/L that extends below the measurement range typical of most conventional CRP assay's. This lower range of measurement may include the evaluation of conditions thought to be associated with inflammation in otherwise healthy individuals.
| Product | Catalog Number | Format | Method |
|---|---|---|---|
| Kit | DZ135A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ135A-CAL | Cal: 4 Level | |
| Control | DZ135A-CON | Con: 3 Level |
Product Features
The Diazyme High Sensitive C-Reactive Protein (hsCRP) Assay has a linear range of 0.20 - 20 mg/L that extends below the measurement range typical of most conventional CRP assay's. This lower range of measurement may include the evaluation of conditions thought to be associated with inflammation in otherwise healthy individuals.
Downloads
Assay Principle
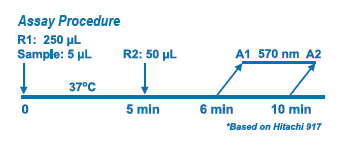
The assay is based on a latex-enhanced turbidimetric immunoassay method. When an antigen-antibody reaction occurs between CRP in a sample and anti-CRP antibody which has been sensitized to latex particles, agglutination results. This agglutination is detected as an absorbance change (570 nm), with the magnitude of the change being proportional to the quantity of CRP in the sample. The actual concentration is then determined by interpolation from a calibration curve prepared from calibrators of known concentration.
Intended use
The Diazyme High Sensitivity C-Reactive Protein (hsCRP) Assay is for the in vitro quantitative determination of C-reactive protein (CRP) in human serum and plasma on automated clinical chemistry analyzers. Measurement of CRP is of use for the detection and evaluation of inflammatory disorders and associated diseases, infection and tissue injury. For in vitro diagnostic use only.
Regulatory Status




