The Diazyme fecal Lactoferrin Assay is a particle-enhanced immunoturbidimetric assay. Lactoferrin in the sample binds to specific anti-Lactoferrin antibodies, which is coated on latex particles, and causes agglutination.
| Product | Catalog Number | Format |
|---|---|---|
| Kit | DZ137A | R1: 1 x 30 mL R2: 1 x 10mL |
| Calibrator | DZ137A-CAL | Cal: 5 x 1 mL |
| Control | DZ137A-CON | Con: 2 x 1 mL |
Product Features
- Particle-enhanced immunoturbidimetric method
- Liquid stable reagent kit, calibrator and control sets offered separately
- Fast test results (10 minutes) for a rapid turnaround time
- Wide range of instrument parameters available for simplifying implementation
Downloads
Informational Articles
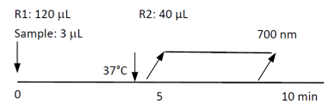
Assay Principle
The Diazyme fecal Lactoferrin Assay is a particle-enhanced immunoturbidimetric assay. Lactoferrin in the sample binds to specific anti-Lactoferrin antibodies, which is coated on latex particles, and causes agglutination. This agglutination is detected as an absorbance change, with the magnitude of the change directly related to the quantity of lactoferrin in the sample. The actual concentration is then determined by the interpolation from a calibration curve prepared from calibrators of known concentration.
Intended use
The Diazyme Lactoferrin Assay is immunoturbidimetric assay used for the quantitative measurement of Lactoferrin in human fecal samples. For Research Use Only in USA. Not for Use in Diagnostic Procedures.


