Diazyme's Pancreatic Elastase Assay is a particle-enhanced immunoturbidimetric assay. Pancreatic Elastase in the sample binds to specific anti-Pancreatic Elastase antibodies, which is coated on latex particles, and causes agglutination.
| Product | Catalog Number | Format |
|---|---|---|
| Kit | DZ134A-KY1 | R1: 1 x 30 mL R2: 1 x 6 mL |
| Calibrator | DZ134A-CAL | Cal: 5 x 1 mL |
| Control | DZ134A-CON | Con: 2 x 1 mL |
Product Features
- Reliable Indicator: The assay accurately measures fecal pancreatic elastase, helping to diagnose pancreatic insufficiency.
- High Sensitivity and Precision: It offers highly sensitive and consistent results for accurate diagnostics.
- Wide Detection Range: The assay detects both low and high pancreatic elastase levels, making it versatile for different patient needs.
- User-Friendly: The ready-to-use reagents and simple sample preparation make the assay quick and easy to perform.
Downloads
Assay Principle
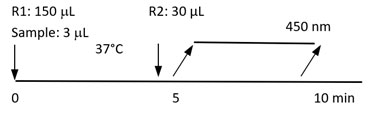
Diazyme's Pancreatic Elastase Assay is a particle-enhanced immunoturbidimetric assay. Pancreatic Elastase in the sample binds to specific anti-Pancreatic Elastase antibodies, which is coated on latex particles, and causes agglutination. This agglutination is detected as an absorbance change, with the magnitude of the change directly related to the quantity of Pancreatic Elastase in the sample. The actual concentration is then determined by the interpolation from a calibration curve prepared from calibrators of known concentration.
Intended use
The Diazyme Pancreatic Elastase Assay is used for the quantitative measurement of human fecal Pancreatic Elastase in human fecal specimens. For in-vitro diagnostic use.
Regulatory Status



